RADIATION
Blogger's note: The information I posted aren't mine. They were all taken during our discussion in our Advanced Physics II class with Mr. Mohamad Ali Ramber and some additional informations from the internet (sources cited at the end of each post). The pictures and animations aren't also mine. Credits to the rightful owners who placed it in google This was the summary of our whole discussions during our second quarter in our class.
Wilhelm Conrad Roentgen -discovered x-rays on 1895. He discovered that x-rays can be generated by directing a cathode ray tube against the wall of a glass tube.
Antoine Henri Becquerel -discovered "radioactivity". He also had serendipitous discovery of the phenomenon "invisible phosphorescence.
Marie Curie -coined the term "radioactivity" and validated Becquerel's "the higher the proportion of uranium in the sample, the more intense the radiation is emitted. She predicted that there's a presence of one or more radioactive elements in the core. She was joined by her husband Pierre Curie. They both isolated two radioactive elements, polonium and radium (2M times more radioactive than uranium).
ALPHA, BETA, GAMMA
Ernest Rutherford found two distinct types of radiation: alpha and beta.
Alpha rays - can be deflected using strong electric and magnetic fields, they were determined to have a charge of +2. Alpha particles are helium nuclei.
Beta rays - determined to have a charge of -1. Beta particles are electrons.
Gamma rays - electromagnetic, like light. It has no charge and no mass. Basically, their photons are the same as light and x-ray photons. However, gamma ray is given to the photons emitted from the nucleus during the gamma decay process.
Ernest Rutherford and Frederic Soddy first proposed that radioactivity produces new elements.
Isotope -the same element but different mass numbers: same number of protons, diffferent number of neutrons.
MECHANISMS OF RADIOACTIVE DECAY
Decay chains/series -decay process in which other radioactive nuclei undergo a series of nuclear transformations before attaining a stable isotope. (e.g. uranium series, thorium series, actinium series, neptunium series)
Half-life -time interval during which half of the atoms originally present disintegrate. It is a fundamental and constant property of a radionuclide. IT cannot be altered by temperature or pressure changes nor by chemical reactions. It is a measure of the stability of the nuclei. A short half-life means an unstable nucleus.
Cosmic Radiation -interaction of cosmic rays with the upper layers of earth's atmosphere.
Terrestrial Radiation -due to the presence of highly radioactive substance rising to the Earth's surface.
Geiger Counter -most common instrument to detect radiation.
Radiation Dose -amount of energy absorbed per kilogram of an irradiated object at the actual target site.
ACUTE vs LONG-TERM EFFECTS
In general, the amount and duration of radiation exposure affects the severity or type of health effect. There are two broad categories of health effects: stochastic (long-term) and non-stochastic (acute/ short-term).
Non-Stochastic/Acute Health Effects
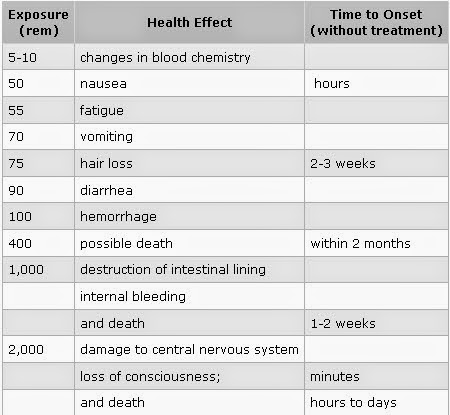
Non-stochastic effects appear in cases of exposure to high levels of radiation, and become more severe as the exposure increases. Short-term, high-level exposure is referred to as ‘acute’ exposure.
Many non-cancerous health effects of radiation are non-stochastic. Unlike cancer, health effects from ‘acute’ exposure to radiation usually appear quickly. Acute health effects include burns and radiation sickness. Radiation sickness is also called ‘radiation poisoning.’ It can cause premature aging or even death. If the dose is fatal, death usually occurs within two months. The symptoms of radiation sickness include: nausea, weakness, hair loss, skin burns or diminished organ function.
Medical patients receiving radiation treatments often experience acute effects, because they are receiving relatively high “bursts” of radiation during treatment.
Stochastic/Long-Term Health Effects
Stochastic effects are associated with long-term, low-level (chronic) exposure to radiation. (“Stochastic” refers to the likelihood that something will happen.) Increased levels of exposure make these health effects more likely to occur, but do not influence the type or severity of the effect.
Cancer is considered by most people the primary health effect from radiation exposure. Simply put, cancer is the uncontrolled growth of cells. Ordinarily, natural processes control the rate at which cells grow and replace themselves. They also control the body’s processes for repairing or replacing damaged tissue. Damage occurring at the cellular or molecular level, can disrupt the control processes, permitting the uncontrolled growth of cells–cancer. This is why ionizing radiation’s ability to break chemical bonds in atoms and molecules makes it such a potent carcinogen.
Other stochastic effects also occur. Radiation can cause changes in DNA, the “blueprints” that ensure cell repair and replacement produces a perfect copy of the original cell. Changes in DNA are called mutations. Sometimes the body fails to repair these mutations or even creates mutations during repair. The mutations can be teratogenic or genetic. Teratogenic mutations are caused by exposure of the fetus in the uterus and affect only the individual who was exposed. Genetic mutations are passed on to offspring.
RADIATION APPLICATIONS
- Food and Agriculture
- Ionizing radiation -mutation, production of new genetic lines of rice, garlic, wheat, etc.
- Food irradiation -form of preservation
- Sterile Insect Technique
- Diagnosis and Therapy
- Radioisotopic Tracing -diagnosis strategy using small amounts of short-lived radioactive isotopes injected into the patient's body.
- Radioactive Dating
- Uranium Dating -determine age of Earth, solar system, moon
- Carbon dating - uses Carbon-16. Determines the age of plants and animal remains.
Albert Einstein predicted the coming of nuclear age through his Theory of Relativity.
NUCLEAR REACTORS
Parts of Nuclear Power Plant
A Nuclear Reactor mainly consists of
a) Fuel
b) Moderators
c) Control rods
d) Shielding
e) Coolant
f) Turbines
g) Generator
h) Cooler Pipes
i) Water Supply

a) Fuel
b) Moderators
c) Control rods
d) Shielding
e) Coolant
f) Turbines
g) Generator
h) Cooler Pipes
i) Water Supply

Fuel:The fissionable material used in the reactor is called as fuel. The commonly used fuels are Uranium, Plutonium or Thorium. It can be U-235, U-238, Pu-236 or Th-232. Uranium is mostly preferred as it has high melting point.
Moderators:Only neutrons of a fairly low speed should be used to have controlled chain reaction. To slow down the speed fast moving neutrons produced during the fission process, moderators are used. Moderator reduces the speed of the neutron by absorbing its energy but not absorb neutron. Graphite, Heavy water and Beryllium are common moderators.
Control Rods:These rods absorb neutrons and stop the chain reaction to proceed further. These are made up of steel containing a high percentage of material like cadmium or boron which can absorb neutrons. When control rods are completely inserted into the moderator block then all the neutrons is absorbed and reaction comes to halt.
Shielding:Shielding prevents radiations to reach outside the reactor. Lead blocks and concrete enclosure that is strong enough of several meters thickness are used for shielding.
Coolant:The coolant is substance in a pipe to the steam generator where water is boiled. This is where heat-exchange process occurs. Heat is absorbed by the coolant that is produced in the reactor. Typical coolants are water, carbon dioxide gas or liquid sodium.
Turbines:Steam produced in the boiler is now passes to a turbine. The force of the steam jet causes the turbine to rotate. Heat energy (steam) is converted to mechanical energy (moving turbine).
Generator:The generator consists of coils that change the mechanical energy into electric energy. The turbine moves and the change in magnetic flux cause electricity. This is transmitted to substations for distribution of
electric power.
Moderators:Only neutrons of a fairly low speed should be used to have controlled chain reaction. To slow down the speed fast moving neutrons produced during the fission process, moderators are used. Moderator reduces the speed of the neutron by absorbing its energy but not absorb neutron. Graphite, Heavy water and Beryllium are common moderators.
Control Rods:These rods absorb neutrons and stop the chain reaction to proceed further. These are made up of steel containing a high percentage of material like cadmium or boron which can absorb neutrons. When control rods are completely inserted into the moderator block then all the neutrons is absorbed and reaction comes to halt.
Shielding:Shielding prevents radiations to reach outside the reactor. Lead blocks and concrete enclosure that is strong enough of several meters thickness are used for shielding.
Coolant:The coolant is substance in a pipe to the steam generator where water is boiled. This is where heat-exchange process occurs. Heat is absorbed by the coolant that is produced in the reactor. Typical coolants are water, carbon dioxide gas or liquid sodium.
Turbines:Steam produced in the boiler is now passes to a turbine. The force of the steam jet causes the turbine to rotate. Heat energy (steam) is converted to mechanical energy (moving turbine).
Generator:The generator consists of coils that change the mechanical energy into electric energy. The turbine moves and the change in magnetic flux cause electricity. This is transmitted to substations for distribution of
electric power.
Sources:
INTERACTIVE PHYSICS NOTEBOOK 2014-2015














No comments:
Post a Comment